The Indian Council of Medical Research (ICMR) told that the rapid antibody-based blood test for the COVID-19 outbreak will come in use by this Wednesday in clusters. Many places have been affected and many lives have been killed by this Coronavirus. The ICMR said that the hotspots are showing a high incidence of confirmed cases.
Head of Epidemiology and Communicable Diseases Division of the ICMR, Raman R Gangakhedkar explained how the laboratory technique combining reverse transcription of RNA into DNA by using real-time reverse transcription-polymerase chain reaction. He also said that the testing for COVID-19 is increasing and soon it will be approaching its full capacity soon.
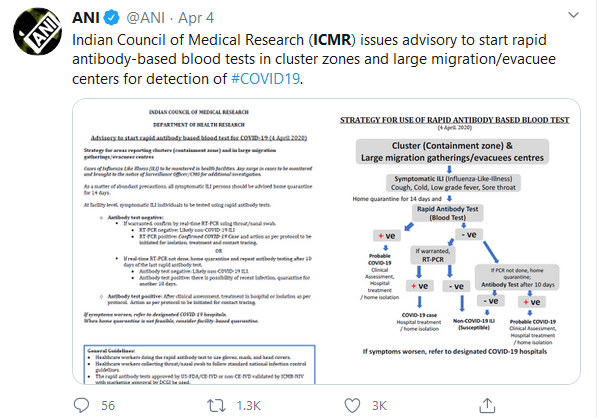
Further, he said, “At the same time, we are expecting delivery of Rapid Test kits (blood-based) for use in response to COVID-19 situation. By Wednesday this should be up and running”, “National Task Force deliberated with experts for ascertaining use of these rapid test kits. We aim to start rapid antibody-based blood test in clusters (with containment zones), and in large migration gatherings/evacuees centers.’’
The Health Ministry has said that all states/UTs have been issued guidelines for implementing the tests and reports will be entered into the ICMR portal which is similar to results of real-time RT PCR tests for COVID-19.
The health Ministry has explained how to test and what will be the scale of testing, that the testing should be focused and judiciously done. The ministry also added that contact-tracing is very much important and now it is greatly enabled by the AarogyaSetu App.
The ministry also gave a statement that the “RT-PCR test detects the virus and the antibody tests, which use blood, detect the body’s response to the virus. A positive result tells that the body was exposed to the virus. The antibody tests, even when used for screening, must be used with care, as with all tests, and interpreted by a professional. As of now, when so used, they can inform how groups of people have been exposed.”
Many experts pointed out that individual-level interpretation is also important and it should be done by a professional, who can take the detailed assessment.
Ramanan Laxminarayan, study author and CDDEP Director and Senior Fellow, said in one of release issued by the group, “Testing samples from multiple patients with a single PCR test, also known as pooled sampling, has been used previously in the early stages of the HIV epidemic when PCR costs were high. Here, we found that the use of a pooled testing strategy could reduce the time, cost, and resources required whilst identifying infected people in a population and estimating the infection rate. This would allow us to identify community clusters for targeted public health interventions.”
Also Read:UPDATE: AIIMS Delhi Doctor Tested Positive With COVID-19
To use this technique, the researchers used mathematical analysis to explore efficient pooling strategies. For a population which contains 256 individual samples, where the maximum samples in a single pool is 64, with 7.3 tests on the average only, it could be possible to differentiate between prevalences of 1% and 5% with a chance of detection of 95% and more chance of false alarm of 4%.
The professor of Texas A&M University said that “This means that rather than testing all 256 individuals in the population, which would be highly costly, with an average of 7.3 tests a 5% prevalence of COVID-19 can be detected using this method.”
